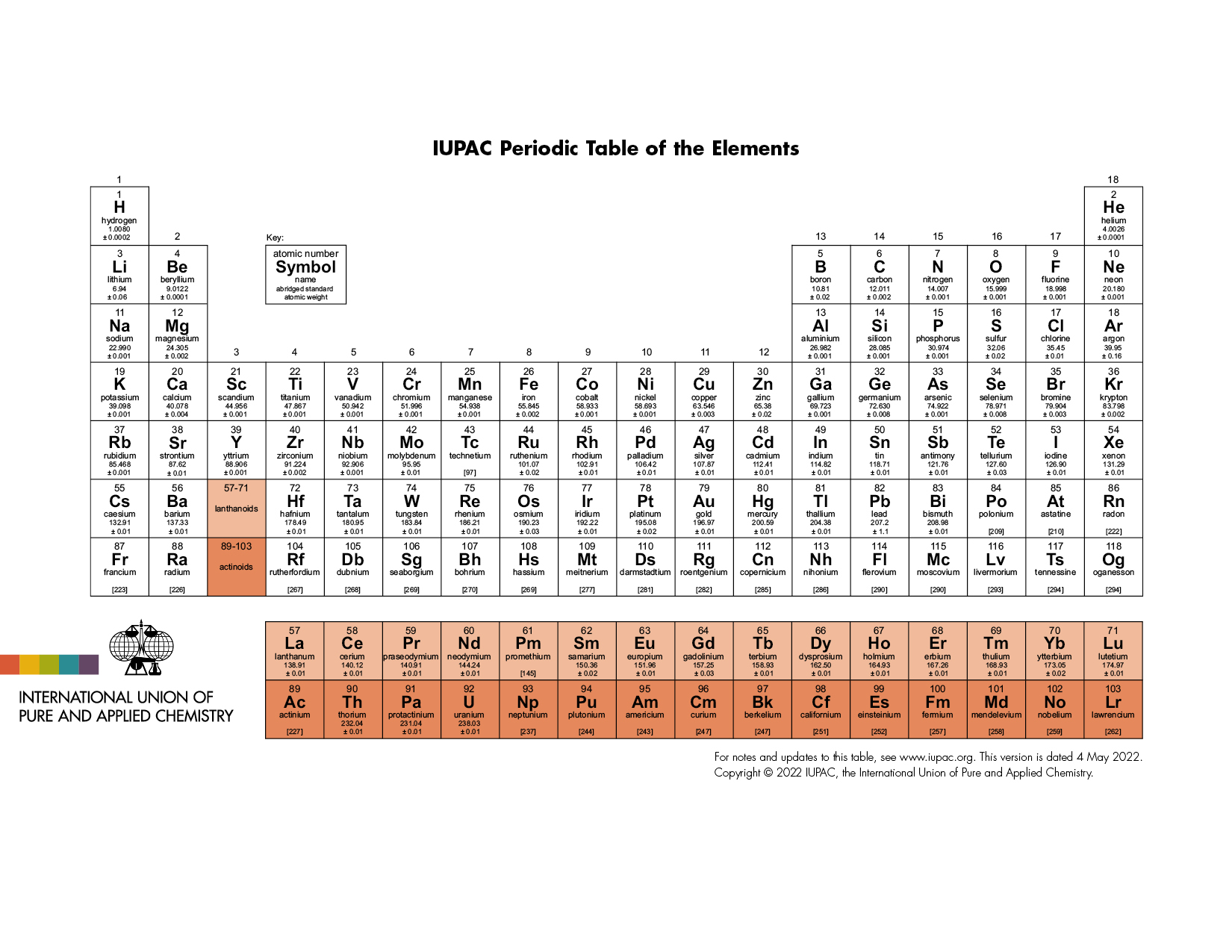
The latest release of the Periodic Table (dated 4 May 2022) includes the most recent abridged standard atomic weight values released by the IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW), compiled as part of the 2021 Table of Standard Atomic Weights 2021. For elements that lack isotopes with a characteristic isotopic abundance in natural terrestrial samples, the mass number of the nuclide with the longest confirmed half-life is listed between square brackets. See PAC (AOP 4 May 2022; https://doi.org/10.1515/pac-2019-0603) for full details or visit Commission II.1 @ciaaw.org
Download the PDF version (letter size or A4) or A3 (PDF) or see earlier versions
Check out SPECIAL Chem Int Jan 2019 — International Year of the Periodic Table (IYPT) — with contributions by Jan Reedijk, Natalia Tarasova, G.J. Leigh, Sigurd Hofmann, Eric Scerri, Juris Meija, Norman E. Holden, Tyler B. Coplen, Peter Mahaffy, Ian Mills, Roberto Marquardt, and more.
 IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Educational Community
IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Educational Community
– Follow IUPAC project 2007-038-3-200 and follow-up project 2014-024-1-200
– Read “Atomic Weights: No Longer Constants of Nature”, Chem Int 33(2), 10–15 (2011), https://doi.org/10.1515/ci.2011.33.2.10
– Explore the interactive version at iupac.org/isotopes-matter (or see release)
– Learn more Why Isotopes Matter! https://iupac.org/100/stories/why-isotopes-matter/
– Review the latest IPTEI element-by-element review including a chart of all known stable and radioactive isotopes for each element and examples practical applications of isotopic measurements and technologies https://iupac.org/iptei/
– Access at http://ciaaw.org/periodic-table-isotopes.htm a full resolution of this Table as PDF (made available by King’s Center for Visualization in Science).
By virtue of its work in relation with the chemical elements, IUPAC can dispense a periodic table that is up-to-date. IUPAC involvement covers various aspects of the table and data that it unveils, and several reports and recommendations, some quite recent, attest of that input.
In particular, IUPAC is directly involved in the following:
- establishing the criteria for a new element discovery
- defining the structure of a temporary name and symbol
- assessing claims resulting in the validation and assignation of an element discovery
- coordinating the naming of a new element, involving the research laboratory and allowing for public comments
- setting up precise rules for how to name a new element
- defining Group 1-18 and collective names
- determining which elements belong to Group 3
- regularly reviewing standard atomic weights
The table is yours to use. Details about the latest release are provided above. Details below provide multiple references to IUPAC journal in Pure and Applied Chemistry (PAC) and magazine Chemistry International (CI).
- Criteria for a new element discovery
Assessing if an element has been “discovered” is not a simple task. While reviewing the discovery profiles of the transfermium elements in the early 90s’, IUPAC and IUPAP set up to establish a series of criteria that must be satisfied for the discovery of an element to be recognized. See details in PAC 1991, Vol. 63, No. 6, pp. 879-886 (https://doi.org/10.1351/pac199163060879) and PAC 1993, Vol. 65, No. 8, pp. 1757-1814 (https://doi.org/10.1351/pac199365081757)
In Nov 2018, a provisional report ON THE DISCOVERY OF NEW ELEMENTS was released by IUPAC/IUPAP. Criteria and guidelines for establishing priority of discovery of potential new elements are presented. — learn more
- Temporary name and symbol
While an element can have been claimed, before the claim has been validated and before the element is formally named, the element has a temporary name and symbol. The pertinent recommendations setting-up that systematic nomenclature was published in 1978; see PAC 1979, Vol. 51, No. 2, pp. 381-384; https://doi.org/10.1351/pac197951020381
In result, that is how, in March 2016, element 113 was called ununtrium or with the symbol Uut.
The story behind the three-letter symbols is recounted in a feature prepared by Lars Öhrström and Norman Holden and published in Chem Int 2016, Vol. 38, No. 2, pp. 4-8; https://doi.org/10.1515/ci-2016-0204
- Validation and assignation of an element discovery
Claims for the discoveries of new elements appear time to time in the scientific literature. IUPAC, along with IUPAP, is involved in assessing these claims. In result, IUPAC technical reports are released that review each pertaining references and recognize the laboratory(ies) whose claims fulfill the agreed criteria.
In 2016, two such reports have been released that cover elements 113, 115, 117, and element 118; See PAC 2016, Vol. 88, No. 1-2, pp. 139–153; https://doi.org/10.1515/pac-2015-0502 and PAC 2016, Vol. 88, No. 1-2, pp. 155–160; https://doi.org/10.1515/pac-2015-0501
- Naming new element
When the discovery of a new element has been validated and the priority for its discovery has been assigned, the naming process can begin. The Laboratory to which the discovery has been assigned is invited to propose a name and symbol. IUPAC will then review the proposal, and if agreed, after an additional 5-month public review, will formalize the name. The most recent example of such recommendations were published in 2012 and for the names and symbols of the elements 114 and 116; See PAC 2012, Vol. 84, No. 7, pp. 1669-1672; https://doi.org/10.1351/PAC-REC-11-12-03
A short review of the current procedures is published in a recent feature by John Corish; See CI 2016, Vol. 38, No. 2, pp. 9-11; https://doi.org/10.1515/ci-2016-0205
On 8 June 2016, IUPAC released the provisional names for the latest 4 elements 113, 115, 117, and 118 – see release and on 28 November 2016, IUPAC announced the approved names and symbols – see release.
For a reflection on the 2016 experience of the naming of elements, see Chem Int Apr 2017, pp. 30-21, by Jan Reedijk; https://doi.org/10.1515/ci-2017-0222
see Archives with earlier compilation and references
- How to name a new element
Here again, IUPAC has a set of guidelines that outline what sort of name an element can bear. Both the root and the ending must be consistent with the agreed recommendations. The detailed recommendations were published in 2002 and a revision published in 2016 to better accommodate element in group 17 and 18. See PAC 2002, Vol. 74, No. 5, pp. 787-791; https://doi.org/10.1351/pac200274050787 and PAC 2016, Vol. 88, No. 4, pp. 401-405 https://doi.org/10.1515/pac-2015-0802 (or https://iupac.org/project/2015-031-1-200)
- Group 1-18 and collective names
Since 1988, IUPAC recommended that the groups (i.e. columns) be simply numbered from 1 to 18. (PAC 1988, Vol. 60, No. 3, pp 431-436; https://doi.org/10.1351/pac198860030431)
Lanthanoids and actinoids are collective names also recommended by IUPAC. Lanthanoids (La to Lu) is preferred over lanthanide, and although lanthanoid means ‘like lanthanum’ and so should not include lanthanum, lanthanum has become included by common usage, however. Actinoids include Ac to Lr.
- Group 3
The question of precisely which elements should be placed in group 3 has been debated from time to time. An IUPAC project has been recently initiated to resolve the question. Will group 3 consist of Sc, Y, Lu, and Lr or, will it consist of Sc, Y, La and Ac?
Stay tune and see https://iupac.org/project/2015-039-2-200 and CI 2016, Vol. 38, No. 2, pp. 22-23; https://doi.org/10.1515/ci-2016-0213
- Standard atomic weights
One of the tasks of the Commission on the Isotopic Abundances and Atomic Weights (CIAAW) is to periodically review atomic-weight determinations. The most recent report “Standard Atomic Weights of the Elements 2021” has been published in PAC in May 2022 (AOP 4 May 2022; https://doi.org/10.1515/pac-2019-0603)
The Commission was established in 1899 (yes, in eighteen ninety nine) and is now operating under the Inorganic Chemistry Division of IUPAC. (see www.ciaaw.org ) It also reviews regularly Isotopic compositions of the elements; the latest compilation is also published in PAC in March 2016 (PAC 2016, Vol. 88, No. 3, pp. 293–306; https://doi.org/10.1515/pac-2015-0503)
Yours to use
While IUPAC has no recommendation for a specific form of the periodic table, i.e. 18-column or 32-column format, the version here presented is in the conventional long form and is yours to use.
Check out earlier versions.